electronic configuration of cr and cu|Electron Configuration for Chromium (Cr, Cr2+, Cr3+) : Tagatay Potassium (K) - Electron Configuration for Chromium (Cr, Cr2+, Cr3+) Time Difference. Hong Kong Time is 7 hours ahead of British Summer Time and 2 hours behind AEST (Australian Eastern Standard Time) 10:00 pm 22:00 in HKT is 3:00 pm 15:00 in BST and is 12:00 am 00:00 in Melbourne, Australia. HKT to BST call time Best time for a conference call or a meeting is between 3pm-6pm in HKT which corresponds to 8am .Welcome to Malik Delgaty's page! Discover the world of this 22-year-old hung muscle stud, model, actor, and dancer based in Montreal. Get ready to have your dreams come true with his explosive content! Home / Malikdelgaty; Meet Malik Delgaty - Your Dream Model. 5 stars 4 stars 3 stars 2 stars 1 star. Get OnlyFans (11.99$)
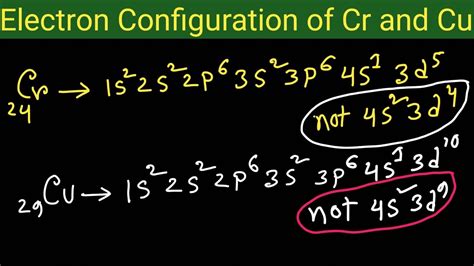
electronic configuration of cr and cu,How to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the configuration for Cr, the ions are .
How to Write the Electron Configuration for Copper (Cu, Cu+, and Cu2+) In order to .
Nitrogen (N) - Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
Magnesium (Mg) - Electron Configuration for Chromium (Cr, Cr2+, Cr3+)

Potassium (K) - Electron Configuration for Chromium (Cr, Cr2+, Cr3+)Sodium (Na) - Electron Configuration for Chromium (Cr, Cr2+, Cr3+)Lithium - Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
Boron is the fifth element with a total of 5 electrons. In writing the electron . The actual electron configurations are: Cr = [Ar] 4s1 3d5. Cu = [Ar] 4s1 3d10. To understand why this occurs, it is important to realize that. 1. Completely filled . Mar 23, 2023 Electronic Configuration of Chromium and Copper Video Lecture from Structure of Atom Chapter of Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.Android Appl.
electronic configuration of cr and cu Electron Configuration for Chromium (Cr, Cr2+, Cr3+)How to Write the Electron Configuration for Copper (Cu, Cu+, and Cu2+) In order to write the Copper electron configuration we first need to know the number of electrons for the .
Rather, Cr and Cu take on half-filled and fully-filled 3d configurations. The electron configuration of chromium (Cr) includes a half-filled 3d subshell. Cr: 1s 2 2s 2 . The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two . In the first row of the transition metals, the ten elements that can be found are: Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron .The Electron Configurations: Exceptions Example 1. Electron Configuration Exceptions to Aufbau's Principle: Configuration of Cr, Cu, Mo, and Ag. Electron Configuration Practice Problems with Step by . This chemistry video tutorial covers exceptions in electron configuration using the examples of Chromium and Copper.electronic configuration of cr and cuHow to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the . The actual electron configurations are: Cr = [Ar] 4s1 3d5. Cu = [Ar] 4s1 3d10. To understand why this occurs, it is important to realize that. 1. Completely filled sublevels are more stable than partially filled sublevels. 2. A sublevel which is exactly half filled is more stable than a partially filled sublevel which is not half full. 3.
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full.Electronic Configuration of Chromium and Copper Video Lecture from Structure of Atom Chapter of Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.Android Appl.
How to Write the Electron Configuration for Copper (Cu, Cu+, and Cu2+) In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom (there are 29 electrons). Once we have the configuration for Cu, the ions are simple.

Rather, Cr and Cu take on half-filled and fully-filled 3d configurations. The electron configuration of chromium (Cr) includes a half-filled 3d subshell. Cr: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 5. The electron configuration of copper (Cu) includes a fully-filled 3d subshell. Cu: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2). In the first row of the transition metals, the ten elements that can be found are: Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu), and Zinc (Zn). Below is a table of the oxidation states that the transition metals can or cannot form.The Electron Configurations: Exceptions Example 1. Electron Configuration Exceptions to Aufbau's Principle: Configuration of Cr, Cu, Mo, and Ag. Electron Configuration Practice Problems with Step by Step Answers. Quantum Chemistry 9.13 - Electron Configuration Exceptions. Electron Configuration Exceptions. Exceptions to Electron Configuration. This chemistry video tutorial covers exceptions in electron configuration using the examples of Chromium and Copper.
How to Write the Electron Configuration for Chromium (Cr, Cr2+, and Cr3+) In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the . The actual electron configurations are: Cr = [Ar] 4s1 3d5. Cu = [Ar] 4s1 3d10. To understand why this occurs, it is important to realize that. 1. Completely filled sublevels are more stable than partially filled sublevels. 2. A sublevel which is exactly half filled is more stable than a partially filled sublevel which is not half full. 3.Electron Configuration for Chromium (Cr, Cr2+, Cr3+) Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full.Electronic Configuration of Chromium and Copper Video Lecture from Structure of Atom Chapter of Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.Android Appl.How to Write the Electron Configuration for Copper (Cu, Cu+, and Cu2+) In order to write the Copper electron configuration we first need to know the number of electrons for the Cu atom (there are 29 electrons). Once we have the configuration for Cu, the ions are simple.
Rather, Cr and Cu take on half-filled and fully-filled 3d configurations. The electron configuration of chromium (Cr) includes a half-filled 3d subshell. Cr: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 5. The electron configuration of copper (Cu) includes a fully-filled 3d subshell. Cu: 1s 2 2s 2 2p 6 3 s 2 3p 6 4s 1 3d 10 The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2). In the first row of the transition metals, the ten elements that can be found are: Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu), and Zinc (Zn). Below is a table of the oxidation states that the transition metals can or cannot form.
electronic configuration of cr and cu|Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH0 · Why do the electron configurations of chromium and copper see
PH1 · Why do the electron configurations of chromium and copper
PH2 · Electronic Configuration of Chromium and Copper
PH3 · Electron Configuration of Transition Metals
PH4 · Electron Configuration for Copper (Cu, Cu+, Cu2+)
PH5 · Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH6 · Electron Configuration Exceptions Examples: Cr, Cu,
PH7 · Electron Configuration Exceptions
PH8 · Electron Configuration Chart of All Elements (Full Chart)
PH9 · 3.3: Electronic Structure of Atoms (Electron Configurations)
PH10 · 1.9: Electron Configurations for Transition Metal Elements